Filgotinib for ulcerative colitis – UPDATED 2020 – FDA approval prospects
Filgotinib may soon become an option for ulcerative colitis patients.
Ulcerative colitis is a type of inflammatory bowel disease that affects about 1 million people in the U.S. It is an autoimmune disease that affects the digestive system, causing inflammation in the innermost lining of the large intestine (colon). Symptoms of ulcerative colitis can include abdominal pain, cramps, rectal bleeding and bloody diarrhea.
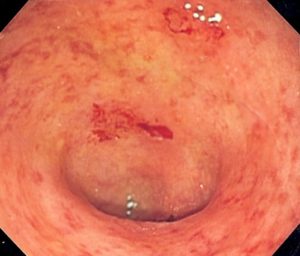
Shown here is the surface affected by ulcerative colitis. Filgotinib is being evaluated as a possible treatment for UC.
This disease can be treated via anti-inflammatory medications and surgery.
Filgotinib is a drug that is being investigated as a possible treatment for ulcerative colitis. It is also being investigated for use in several other conditions, including Crohn’s disease, rheumatoid arthritis, Sjögren’s syndrome, uveitis, ankylosing spondylitis, lupus membranous nephropathy, cutaneous lupus erythematosus and psoriatic arthritis.
The drug was initially developed by Belgian-Dutch pharmaceutical and biotech firm Galapagos NV.
Filgotinib FDA Approval
Currently, filgotinib is still an investigational agent, as it has not yet received approval from the U.S. Food and Drug Administration (FDA) or any other recognized medical regulatory body. As such, it is not available on the market.
But that could soon change.
Filgotinib is a drug developed by Galapagos NV.
Testing is farthest along in rhematoid arthritis patients. Galapagos and another company, Gilead, have been working together to present filgotinib as a safe and effective drug to the FDA. Trials have suggested that filgotinib “is better than methotrexate alone while suggesting it has an edge over AbbVie’s Humira” for rheumatoid arthritis, FierceBiotech reports.
Gilead has also filed for approvals of the drug in Japan and Europe. Filgotinib has been accepted for a marketing review by the European Medicines Agency (EMA). It’s anticipated that approvals will start to come through in the second half of 2020, Fierce Biotech reports.
Applications for approval are based on phase 3 testing of the drug in nearly 3,500 rheumatoid arthritis patients. FierceBiotech reports that the data made available seem likely to win approval for the drug. What’s less clear is whether filgotinib will have clear enough advantages over competing drugs to yield blockbuster sales.
But as noted, filgotinib is being tested for other conditions as well. The drug showed promising results in a recent trial in 1,348 ulcerative colitis patients. At a 200 milligram dose, it led to remission at a higher rate than a placebo did, according to the research.
The findings suggest that the drug could lead to “meaningful and sustained improvement in treatment response with an oral therapy,” said Merdad Parsey, chief medical officer for Gilead Sciences.
Gilead hopes that within four years the drug will be marketed for five disease indications.
Filgotinib Side Effects
Much of the buzz around filgotinib has centered on its promising safety profile. Other drugs in its class — “oral, selective JAK inhibitors” — have raised safety concerns, MarketWatch reports.
Filgotinib is what’s known as a “highly selective JAK1 inhibitor.” JAK inhibitors, also known as Janus kinase inhibitors, are a newly discovered way of treating inflammatory and autoimmune diseases because of their capacity to block signals across multiple cytokines.
Two well-known examples of JAK inhibitors are Xeljanz, from Pfizer, and Olumiant, from Eli Lilly. MarketWatch reports that they are sometimes known to cause serious side effects such as increased chance of infection and cancer. In addition, MarketWatch reports that Olumiant has been linked to cardiovascular troubles.
In research on rheumatoid arthritis patients, filgotinib has been to shown to be as effective as competing drugs and to have a safety profile that is encouraging, according to MarketWatch.
Filgotinib Cost
Cost has long been a concern when it comes to treatment for chronic conditions such as ulcerative colitis, Crohn’s disease and rheumatoid arthritis. In the U.S., insurance plans typically cover treatment for these diseases, but in many cases they do not pay 100 percent of costs, and patients must pay the remainder themselves.
As filgotinib has not yet hit the market, it’s not clear what its cost may be. Fierce Pharma reports that overall sales of the the drug could hit $1.28 billion in 2024.
Filgotinib will be a competitor to upadacitinib, which AbbVie markets as Rinvoq. That drug won FDA approval for treatment of rheumatoid arthritis in August 2019.
The price for a year’s worth of upadacitinib is approximately $59,000, according to Reuters. AbbVie said it was making available a co-pay card and support program so that eligible patients with private insurance could get the drug for $5 a month. A different assistance program from the company would make upadacitinib available for uninsured and underinsured patients.
Filgotinib vs. Upadacitinib
These two drugs are extremely similar, with the same mechanism for treating the same set of diseases. Upadacitinib is a drug manufactured by AbbVie, which was initially a shareholder in the development of Filgotinib with Galapagos NV, but pulled out later in September 2015.
Upadacitinib
It was then that Galapagos associated themselves with Gilead to have a partner in the development and promotion of the drug.
Another difference between the two is that Upadacitinib has already won the race in securing the approval of FDA for usage of the public. This might have given Upadacitinib a head start in the market, but who will come up as the leader of the market in the future will be decided once Filgotinib gets the approval from FDA and enters the market.


